Research
Solid State Ionics
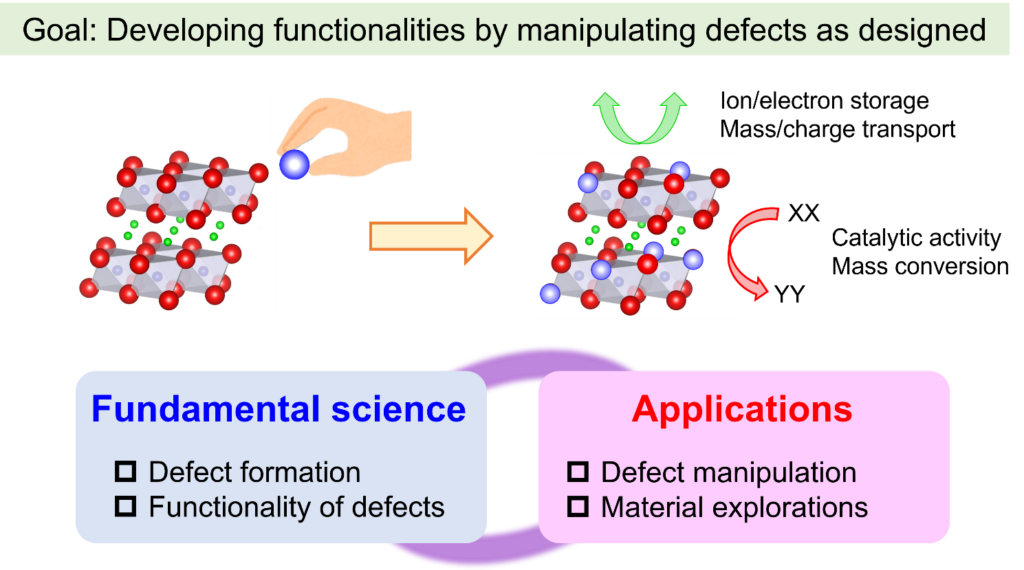
It is generally accepted that some solid materials can achieve high ionic conductivity by appropriate design and control of defect structures. Solid state ionic materials have been applied in energy storage and conversion devices such as rechargeable batteries, fuel cells, electrolysis cells and catalysts, and further advancement is desired as these materials play a crucial role in energy storage/conversion devices. “Solid State Ionics” is a field of material science which deals with ion conduction in solids and the related electrochemical phenomena. This fundamental science provides important guidelines that accelerate the aforementioned energy-devices. Our group uses Solid State Ionics as a fundamental science and is tackling various research topics, battery technology, catalysts, and the development of new synthesis techniques.
High energy density batteries ~Electrode materials~
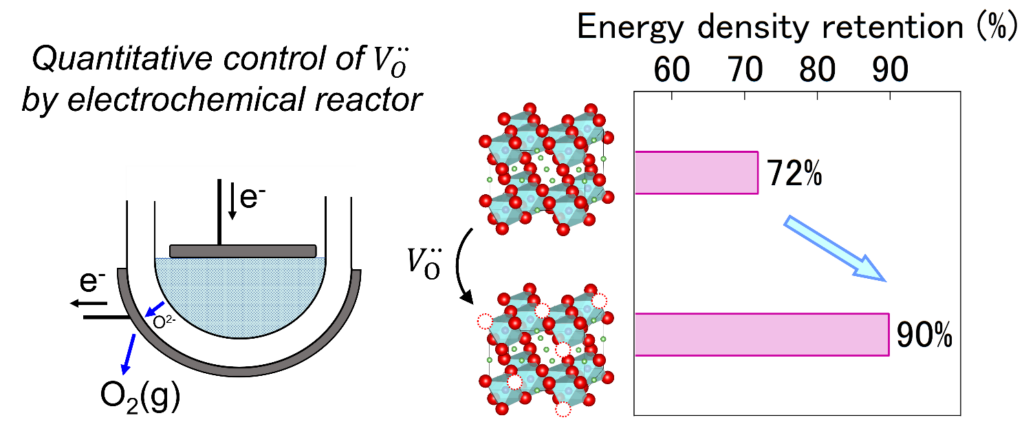
Lithium-ion batteries are used as power sources for smartphones, laptops, and electric vehicles. While lithium-ion batteries support modern society, there is a need for further improvements in energy density, safety, and reliability. Therefore, needs for next-generation batteries are growing, such as advanced lithium-ion batteries, solid-state batteries, and sodium-ion batteries. Batteries are generally composed of electrode materials (both positive and negative electrodes) with energy storage capabilities, electrolytes with high ionic conductivity, and separators. Our group focuses on developing electrode materials that achieve high energy density with excellent reliability based on Solid State Ionics, Defect Chemistry and Inorganic Solid Chemistry. We are conducting unique research, distinct from conventional battery studies, to understand the role of defect structures in battery materials.
Efficient mass/energy conversion technologies ~Electrocatalysts~
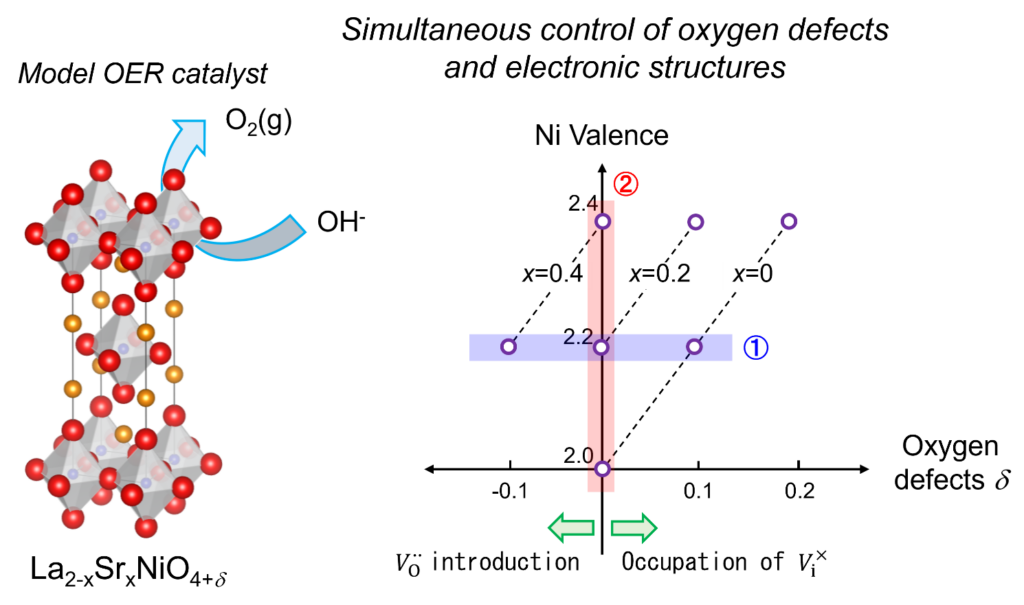
Hydrogen is considered one of the ultimate clean energy sources, but there remain many technological challenges related to its production, storage, and transportation. Our group focuses on hydrogen production through water electrolysis, specifically addressing the major technological challenge of developing oxygen evolution reaction (OER) catalysts. In water electrolysis, hydrogen is produced at the anode, and oxygen is produced at the cathode, with the rate of the overall electrolysis reaction being determined by the oxygen evolution reaction. Therefore, enhancing the oxygen evolution reaction directly contributes to improving the efficiency of hydrogen production, necessitating the development of high-performance OER catalysts. We are working on elucidating the reaction processes that determine catalytic properties based on solid ionics, and developing electrolytic catalysts based on these findings.
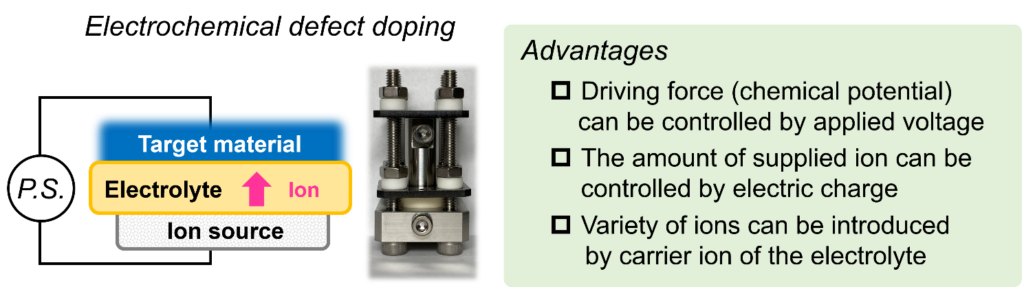
Development of electrochemical defect control techniques
To address environmental issues and achieving the SDGs, developing inorganic functional materials is an extremely important technological challenge. This requires not only the advancement of existing synthesis technologies but also the creation of innovative new synthesis techniques that can reach beyond the limit of current synthesis technologies. Our group has conceived the idea of using electrochemical cells, composed of ionic conductors, as reactors for material synthesis. This technique has some advantages compared with conventional techniques, 1) establishing extreme reaction conditions (e.g. ultra-high chemical potential environments) is possible by simply applying external voltage, 2) the amount of supplied ion can be controlled by electric charge, and 3) variety of ionic species can be introduced by changing electrolyte. This approach is expected to bring significant advancements to the field of materials science such as exploration of new materials, enhancement and/or the addition of functionalities.